Putting POMBILITI + OPFOLDA Into Action
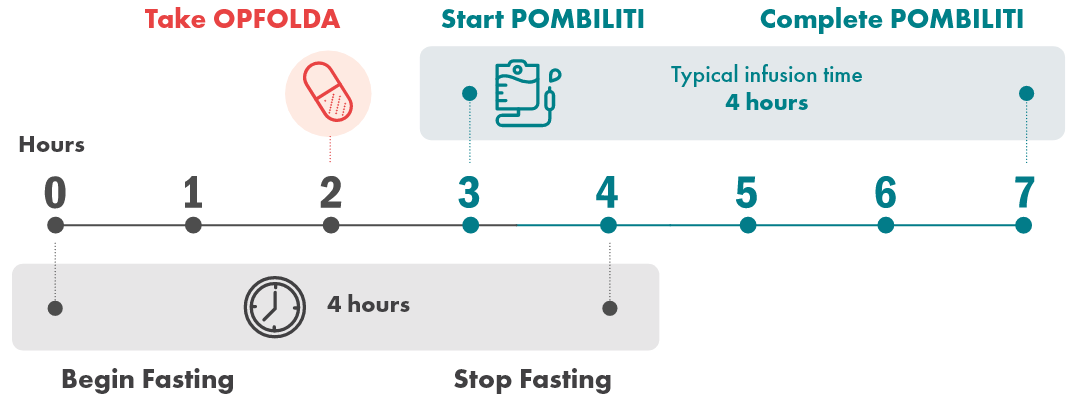
A typical infusion day looks like this:
POMBILITI must be administered in combination with OPFOLDA1,2
Getting started
- Prior to POMBILITI administration, consider pretreating with antihistamines, antipyretics, and/or corticosteroids, especially if premedication was used with the patient’s previous ERT1
- Recommended dosage for POMBILITI: 20 mg/kg of actual body weight administered intravenously every other week1
- Recommended dosage for OPFOLDA2*:
- For patients weighing ≥50 kg, 260 mg (4 capsules of 65 mg) administered orally every other week
- For patients weighing ≥40 kg to <50 kg, 195 mg (3 capsules of 65 mg) administered orally every other week
*
For recommended dosage in patients with renal impairment, see Section 2.3 of the OPFOLDA full Prescribing Information, or see the OPFOLDA recommended dosage section below.

Taking OPFOLDA1,2
- OPFOLDA should be taken approximately 1 hour before intravenous infusion of POMBILITI
- OPFOLDA capsules should be swallowed whole 2 hours after the last meal or snack. Unsweetened beverages (eg, water, tea, or coffee with no cream, sugar, or sweeteners) are permitted between meals. Patients should not consume other beverages or food for at least 2 hours prior and 2 hours after taking OPFOLDA. Patients can eat and drink as usual 2 hours after taking OPFOLDA
- If the OPFOLDA dosage is missed, POMBILITI should not be administered and treatment should be rescheduled at least 24 hours after OPFOLDA was last taken

Starting POMBILITI1
- POMBILITI must be reconstituted and diluted prior to use
- The POMBILITI infusion should begin approximately 1 hour after oral administration of OPFOLDA
- If the POMBILITI infusion cannot be started within 3 hours of oral administration of OPFOLDA, reschedule POMBILITI and OPFOLDA at least 24 hours after OPFOLDA was last taken
- If POMBILITI in combination with OPFOLDA are both missed, restart treatment as soon as possible
For female patients:
- POMBILITI in combination with OPFOLDA is contraindicated in pregnancy
- Verify the pregnancy status in females of reproductive potential prior to initiating treatment with OPFOLDA in combination with POMBILITI
- Advise females of reproductive potential to use effective contraception during treatment with OPFOLDA in combination with POMBILITI and for at least 60 days after the last dose
POMBILITI dose is determined by actual body weight1
Calculating the POMBILITI dose
Recommended dosage for POMBILITI is 20 mg/kg of actual body weight administered every other week. Patients should be weighed at every visit to calculate the correct dose.
1

Patient’s actual body weight (kg)
x
Recommended dosage (20 mg/kg)
=
Patient’s dose (mg)
2
Patient’s dose (mg)
÷
105 (mg/vial)
=
Number of vials to reconstitute
If the number of vials includes a fraction,
round up to the next whole number.
Never round down.
Note that each vial requires 7.2 mL of sterile water to reconstitute, but should only yield 7.0 mL of solution for the infusion bag.
Example:
A 75-kg patient dosed at 20 mg/kg
- Step 1: Patient’s dose (mg), calculated as 75 kg x 20 mg/kg = 1500 mg total dose
- Step 2: Number of vials to reconstitute, calculated as 1500 divided by 105 mg/vial = 14.29 and round up to 15 vials
- Step 3: Extraction volume per vial is 7.0 mL. 14.29 vials x 7.0 mL = 100.0 mL total extraction volume (2 mL from final vial)
Dosing calculator
to be withdrawn
Use this calculator to confirm your calculations for patients weighing between 40 and 140 kg (88 to 309 lbs). For more information about dosing and administration, please refer to the full Prescribing Information for POMBILITI.
By using this tool, you agree to the following:
This dosing calculator is provided “as is” and is not intended to be used as a substitute for clinical judgment. All calculations should be confirmed before dosing. This guide is intended for use only by healthcare professionals. Amicus Therapeutics makes no claims as to the accuracy of the information and shall not be liable for any decisions made or actions taken in reliance on this information.
In calculating the dose, the number of vials is always rounded up, not down. A partial dose is withdrawn from the final vial.
This calculator is intended for US HCPs only and should not be used by HCPs located outside the US.
Determining the OPFOLDA dose
- OPFOLDA is administered orally and can be given with other pre-infusion medications
- Only use OPFOLDA capsules in conjunction with POMBILITI
- OPFOLDA is administered orally every other week. The recommended dosage is based on actual body weight:
- For patients weighing ≥50 kg, the recommended dose is 260 mg (4 capsules of 65 mg)
- For patients weighing ≥40 kg to <50 kg, the recommended dose is 195 mg (3 capsules of 65 mg)
- Recommended OPFOLDA dosage in patients with moderate or severe renal impairment§:
- ≥50 kg = 195 mg (3 capsules of 65 mg)
- ≥40 kg to <50 kg = 130 mg (2 capsules of 65 mg)
For patients with mild renal impairment, the recommended OPFOLDA dosage is the same as for patients with normal renal function.
§Renal function is classified by creatinine clearance (CLcr) based on the Cockcroft-Gault equation. Mild renal impairment is CLcr 60-89 mL/min, moderate renal impairment is CLcr 30-59 mL/min, and severe renal impairment is CLcr 15-29 mL/min.
Administering the infusion
- Infusion of POMBILITI should start approximately 1 hour after oral administration of OPFOLDA
- The infusion will take approximately 4 hours
- If the infusion cannot be started within 3 hours of administration of OPFOLDA, reschedule treatment to at least 24 hours after OPFOLDA was last taken. If both medications are missed, restart treatment as soon as possible
- The infusion solution should be administered at room temperature
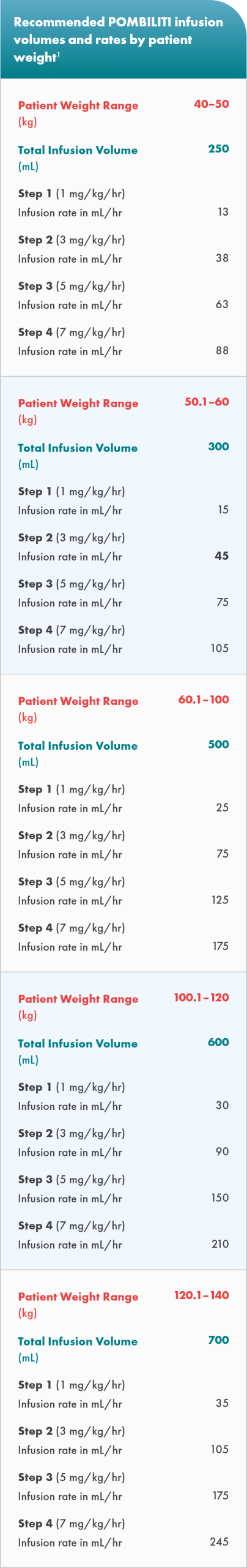
- The initial recommended infusion rate is 1 mg per kg per hour for the first 30 minutes
- The infusion rate may be gradually increased by 2 mg/kg per hour every 30 minutes, if there are no signs of hypersensitivity or infusion-associated reactions (IARs), until a maximum rate of 7 mg/kg/hour is reached; then, maintain the infusion rate at 7 mg/kg/hour until the infusion is complete
Preparing the infusion1
Reconstituting the lyophilized powder
1

Remove vials from the refrigerator and set aside for approximately 30 minutes to allow them to come to room temperature.
2
Reconstitute each vial by slowly injecting 7.2 mL of sterile water for injection down the inside wall of each vial to avoid foaming.
Avoid forceful impact of sterile water for injection on the lyophilized powder and avoid foaming.
3
Roll and tilt each vial to allow the
lyophilized powder to dissolve completely.
This typically takes 2 minutes. Do not invert, swirl, or shake.
4

Acceptable:
Clear to opalescent, colorless to yellowish, essentially particle free.
Visually inspect the reconstituted vials for particulate matter and discoloration.
The reconstituted solution appears as a clear to opalescent, colorless to yellowish solution, essentially particle free.
- Discard if foreign matter is observed or the solution is discolored
- Each reconstituted vial should yield a concentration of 15 mg/mL
If the reconstituted POMBILITI vials are not used immediately, store refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours. Do not freeze.
Diluting the solution
1
Remove airspace within a bag of 0.9% sodium chloride injection.
- Remove an equal volume of 0.9% sodium chloride injection that will be replaced by the total volume (mL) of reconstituted POMBILITI
2
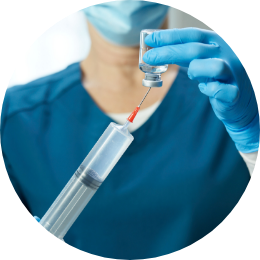
Slowly withdraw 7 mL of reconstituted solution from each of the vials until the patient’s dose is obtained.
- Discard any remaining reconstituted solution in the last vial
3
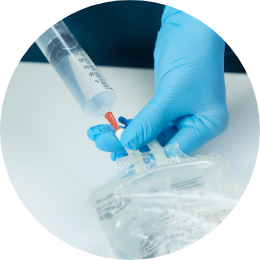
Slowly inject the reconstituted POMBILITI directly into the infusion bag.
- POMBILITI should be administered as stated in the Prescribing Information
- An intravenous administration set should be used with an inline, low protein-binding, 0.2-micron filter. If the intravenous line blocks during infusion, change the filter
- If the diluted solution is not administered immediately, store refrigerated at 2°C to 8°C (36°F to 46°F) for up to
16 hours. Storage at room temperature is not recommended. Do not freeze - Other medicines should not be infused in the same intravenous line as the diluted POMBILITI solution
Safe storage of POMBILITI
Storage before reconstitution
Store at 2°C to 8°C
(36°F to 46°F)
Do not freeze
Store in the original packaging
to protect from light
POMBILITI is supplied as a sterile, nonpyrogenic, white to slightly yellowish lyophilized cake or powder for reconstitution with sterile water for injection to yield a concentration of 15 mg/mL, then further diluted with 0.9% sodium chloride for injection for intravenous infusion. Single-use vials are available in 105-mg dosage only.
Storage after reconstitution and dilution1

‡After removal of the diluted solution from the refrigerator, completely infuse within 6 hours and do not restore in the refrigerator.
If the solution is not administered immediately, it must be refrigerated.
Do not freeze the reconstituted vial or the diluted POMBILITI solution in the infusion bag.
OPFOLDA storage
Storage for OPFOLDA capsules2
OPFOLDA is supplied as a hard gelatin capsule containing 65 mg of miglustat
-
- Do not use if inner seal is missing or broken
- Keep out of reach of children
- Store in the original container or equivalent to protect from light
Sign up for emails
Sign up to receive updates about LOPD and POMBILITI + OPFOLDA
ERT, enzyme replacement therapy; CLcr, creatinine clearance; IARs, infusion-associated reactions.
References: 1. POMBILITI. Prescribing information. Amicus Therapeutics US, LLC; 2024. 2. OPFOLDA. Prescribing information. Amicus Therapeutics US, LLC; 2024.