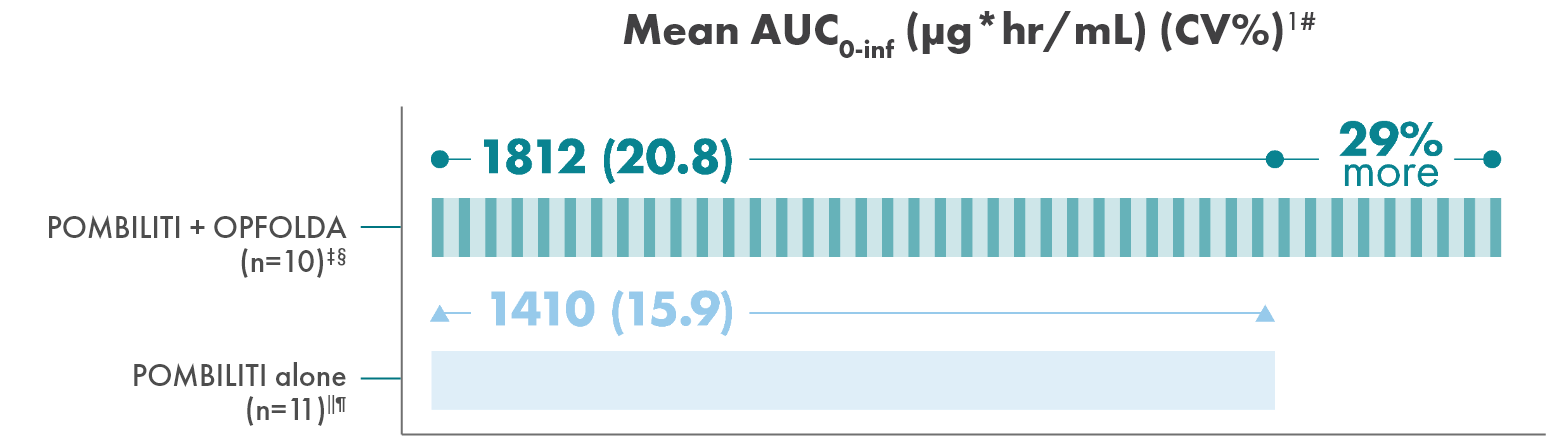The Power of Plus
+
*Enzyme derived from a Chinese Hamster Ovary (CHO) cell line using perfusion methodology, resulting in cellularly (CHO)-derived N-glycans.1
POMBILITI + OPFOLDA was developed to address
3 key challenges:

Maximizing Uptake
to the lysosome with bis-M6P1

Increasing Enzyme Activity
in the lysosome6

Improving Stability
in the blood stream6
Designed to maximize enzyme uptake1
Developed to address key challenges in delivering rhGAA with a naturally derived*
bis-M6P–enriched enzyme and stabilizer1,4†
Increase Active Enzyme in the Blood6
OPFOLDA binds with and stabilizes POMBILITI in the blood and increases the amount of active enzyme that can reach the muscle3,6
Maximizing Uptake1
POMBILITI, a naturally derived* bis-M6P–enriched enzyme, in combination with OPFOLDA, binds with high affinity to CI-MPRs on the cell surface to be transported to the lysosome1,6
Complete Processing in the Lysosome1
Once inside the lysosome, OPFOLDA disassociates from POMBILITI3
POMBILITI is completely processed into the most active form of GAA, like endogenous GAA7-9
POMBILITI in its most active form cleaves glycogen into glucose1
*Enzyme derived from a Chinese Hamster Ovary (CHO) cell line using perfusion methodology, resulting in cellularly (CHO)-derived N-glycans.1
†Based on in vitro data.
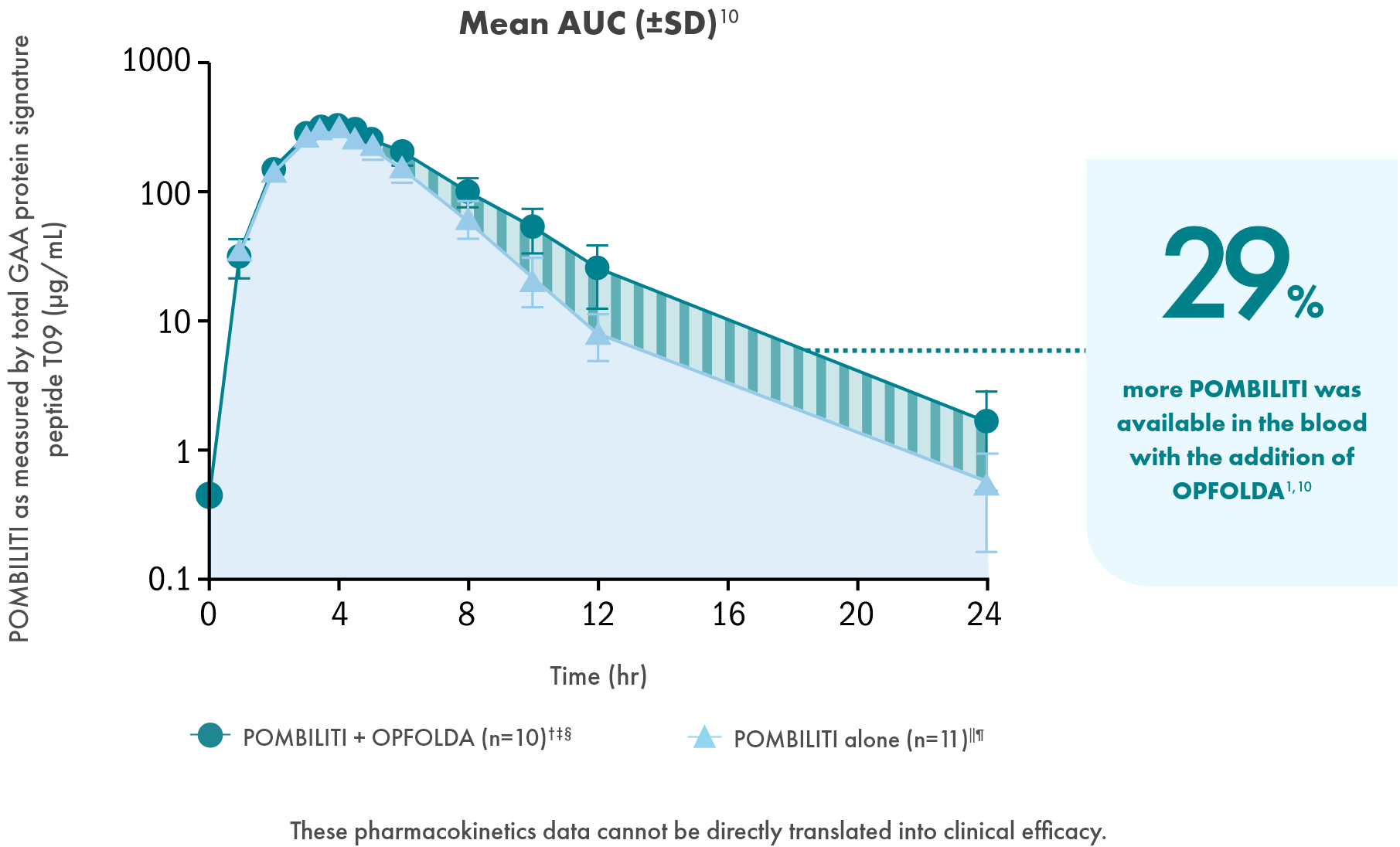
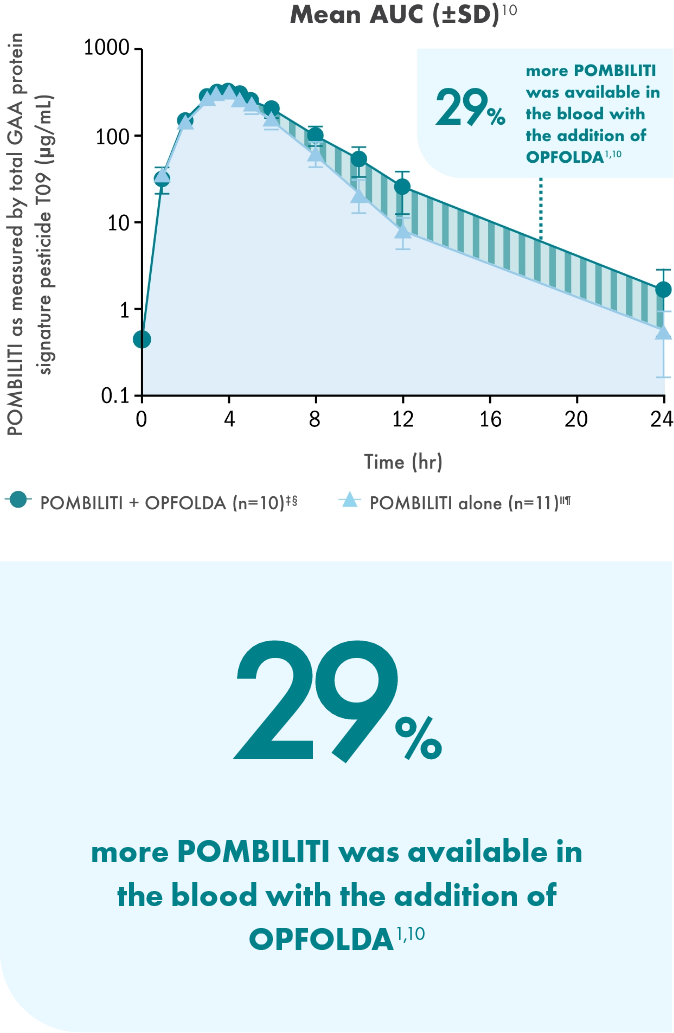
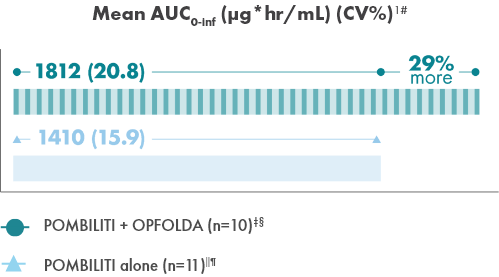
Adding OPFOLDA increased the availability of POMBILITI in
the blood1
Pharmacokinetics of POMBILITI in the blood in ERT-experienced adults with LOPD
AUC, area under the curve; CV, coefficient of variation.
† One subject was not dosed properly with OPFOLDA and was excluded from the analysis.10
‡ POMBILITI 20 mg/kg + OPFOLDA 260 mg.1
§ 20 mg/kg.1
‖ POMBILITI is not approved for use without OPFOLDA.1
¶ AUC0-inf is area under the curve (from time 0 to infinity), representing total drug exposure over time.1,11
Sign up for emails
Sign up to receive updates about LOPD and POMBILITI + OPFOLDA
AUC, area under the curve; bis-M6P, bis-phosphorylated mannose 6-phosphate; CI-MPR, cation-independent mannose 6-phosphate receptor; CV, coefficient of variation; ERT, enzyme replacement therapy; GAA, acid alpha-glucosidase; LOPD, late-onset Pompe disease; M6P, mannose 6-phosphate; rhGAA, recombinant human acid alpha-glucosidase.